We are a
human-sized consultancy &
full-service CRO
—delivering seamless solutions in strategy, regulatory authorization, and commercialization for your innovation in Europe and internationally.
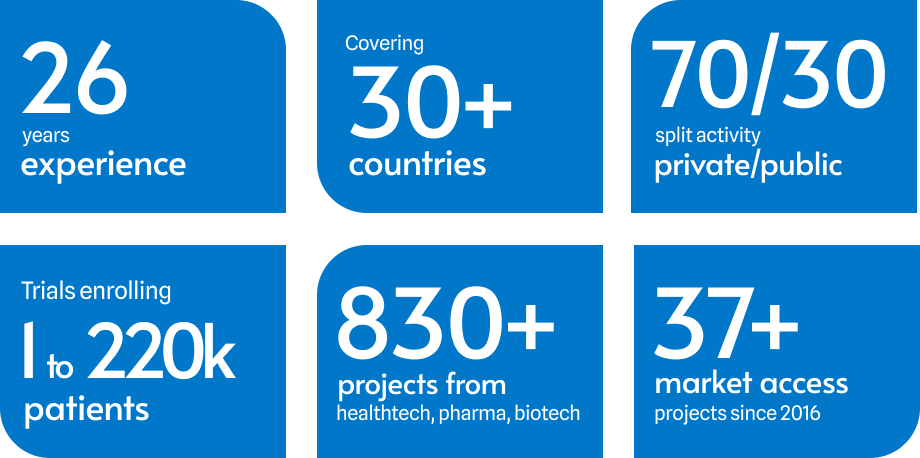
ClinSearch is a French Clinical Research Organization (CRO) specializing in clinical development and commercialization services for medical device and drug development across Europe. We provide tailored, cost-effective solutions for managing your pre- or post-marketing study in Europe, and securing reimbursement in key markets. We offer comprehensive services including study methodology development, document writing and review, regulatory and site feasibility, adapted electronic data management solutions, monitoring in Europe, and top-notch study and statistical reports—all under one roof in Malakoff, South-West Paris. We have been serving the pharmaceutical and medical device industry since 1999, earning a reputation for delivering practical, measurable and high added-value data.

About us
At ClinSearch, excellence is our standard.
We are committed to offering the highest quality services and strategic advice, grounded in scientific rigor and unwavering regulatory compliance.
From early-stage development to post-marketing studies, our mission is to empower your innovation with tailored, practical, and cost-effective solutions. We partner closely with you to navigate the complexities of clinical research and market access with precision, integrity, and results in mind.
Our promise: to provide clear, actionable guidance, identify the most efficient path forward, and deliver results on time and on budget.
Our brands & services
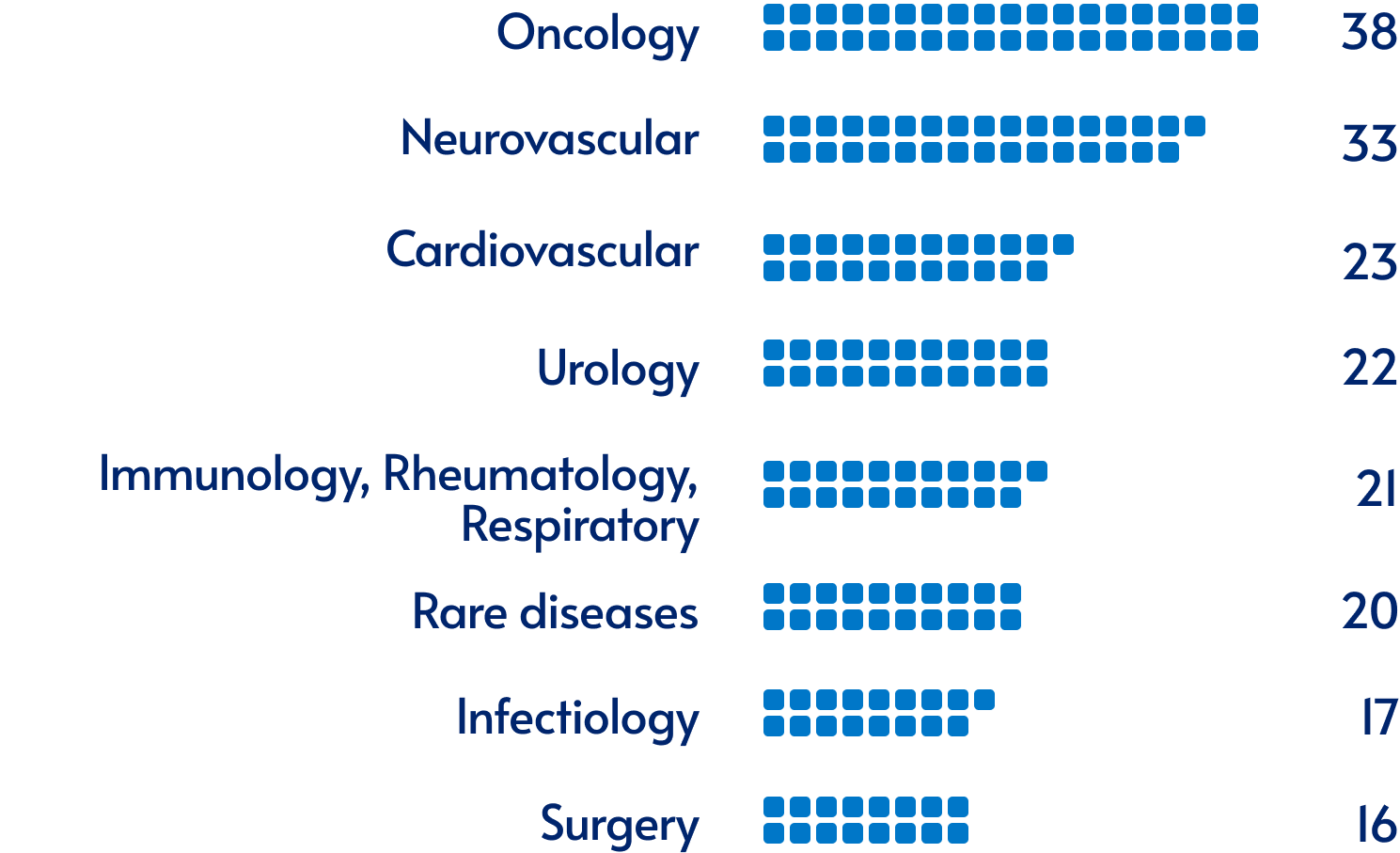
Our activity
Our team
A proud employer of 21 nationalities, with an uncompromising focus on best industry practices, we are able to mobilise our agile team of cross-functional experts to overcome any challenge in your clinical development or market access project.
Our services are always evolving, recently expanding into digital solutions for accelerated recruitment and generation of superior clinical evidence and data to support reimbursement.
